About TRREE
TRREE stands for Training and Resources in Research Ethics Evaluation.
TRREE is headed by a consortium of interested persons from Northern and Southern countries. It aims to provide basic training, while building capacities, on the ethics of health research involving humans so that research meets highest standards of ethics and promotes the welfare of participants. TRREE achieves this goal primarily by developing a training programme with local collaborators. In its initial stages TRREE focused primarily, but not exclusively, on the needs of African countries.
TRREE provides free-of-charge access to:
- e-Learning: a distance learning program and certification on research ethics evaluation
- e-Resources: a participatory web-site with international, regional and national regulatory and policy resources
TRREE’s learning material is currently available in English [EN], French [FR], German [DE] and Portuguese [PT].
The e-learning programme is based on internationally recognized ethical principles and regulations. It integrates local issues and perspectives from low-and middle-income countries, most notably from African countries, that are relevant to all those who must ensure the protection of research participants and who promote highest ethical standards.
The ongoing development of this programme promoted co-learning, collaboration and capacity-building amongst partners.
Objectives
TRREE has three general objectives :
- First, to increase knowledge as well as practical skills of those involved in the management and conduct of ethics evaluation and research partnerships.
- Second, to create a participatory process that will nourish lasting partnerships with and amongst African as well as other low-and middle income partners.
- Third, to create a resource that will facilitate the dissemination of knowledge in North-South partnership. Overall, this will strengthen the research ethics evaluation capacities in African, European and other participating countries.
Approach
The primary goal of these training modules is to provide training and resources to those who ensure the protection of the rights and interests of individuals and communities serving as participants in health research. The training material is designed for all those involved in collaborative research involving humans including physician-investigators and other researchers, students, research ethics committees and regulatory agencies.
The modules are based on well-established principles of research ethics, as expressed in documents such as the Declaration of Helsinki. Research ethics operates within the universal human rights framework as elaborated in the Universal Declaration of Human Rights (1948), the Convention on the Rights of the Child (1989),and other international human rights instruments. Research on humans often involves risks as well as potential benefits. Research risks are mostly borne by the research participants. So it is important to ensure that their interests are respected and their well-being is protected. Many actors have a collaborative role to play in this.
Within this framework, researchers bear the primary responsibility for the safety and wellbeing of research participants. Researchers fulfil this responsibility during the writing of protocols, the conduct of research involving humans and by acting with integrity. The process of research ethics evaluation by Research Ethics Committees (RECs) is also directed at protecting research participants. Ensuring appropriate protection of research participants while not unduly limiting potentially promising health research requires awareness of local and international guidelines and critical assessment and thinking.
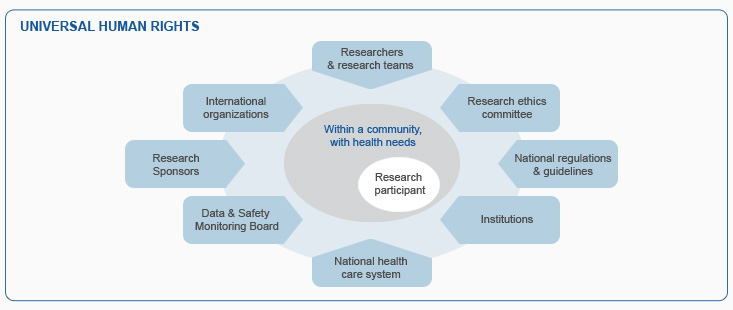
Audience
TRREE provides training and resources that are relevant to the work of all those who must ensure the protection of the interests and well-being of humans who participate in research as well as the promotion of the highest ethical standards. While some modules may focus on more specific training needs of research ethics committee members, or research teams including investigators, nurses, or study coordinators, the training is open to all and may be of interest to health authorities, funding agencies and universities, as well as to political authorities, patients and the media.
Certificate
Modules 1, 2 and 3 have questions integrated to the training material. Participants need to give the correct answer to move to the next section of the module.
At the end of each module, a certificate is delivered to participants who got 70% of correct answers on their first click (first try).
Fee for the Delivery of the Good Clinical Practice (GCP) module (4) TRREE Certificate
TRREE’s philosophy is based on the right to education and provides free on-line access to e-learning modules. However, the maintenance of the programme and the steady control of its quality, in particular through a regular update of its content, are not without a cost. In order to sustain and bolster the programme, we are therefore introducing a 50 Swiss Francs fee for the delivery of the official Certificate for the Good Clinical Practice (GCP) module (4). This amount will be collected only from participants from high income economies (as identified in the 2016 World Bank’s list). We thank you for your understanding and your support that will allow us to continue providing a high quality, accurate and up-to-date training programme in research ethics and regulation worldwide.
Access to the training module itself remains free. Only the delivery of the GCP module Certificate will be charged.
TRREE certificates are formally acknowledged by the following organizations:
 |
 |
Copyright
© 2009, Dominique Sprumont & Clement Adebamowo, Jérôme Ateudjieu, Cédric Baume, Charles Becker, Marie-Charlotte Bouësseau, Ogobara Doumbo, Marie Hirtle, Joyce Ikingura, Wen Kilama, Dirk Lanzerath, Sébastien Lormeau, Peter Ndumbe, Alassane Niaré, Aceme Nyika, Jean-François Perret, Marcel Tanner, Douglas Wassenaar, John R. Williams
Disclamor
Legal texts and policies are provided for reference purposes only – documents should not be considered official versions
ACKNOWLEDGMENTS
The TRREE team wishes to warmly thank the following people for their support:
Administrative support
- Dominique Mengisen (Switzerland)
- Rolf Klappert (Switzerland)
Graphic Design
- Pixailes Design Graphique (Canada)
- Hughes Richartzagen (Belgium)
- Valérie Seydoux (Switzerland)
As well as every person who contributed to the needs assessment and the drafting of this training program, in particular the chairpersons and the members of the Research Ethics Committees in Mali, Cameroon, Tanzania, and Switzerland.
This website is powered by:
© 1999 onwards, Martin Dougiamas under the GNU GPL.
IT Consultant, web developer & hosting:
